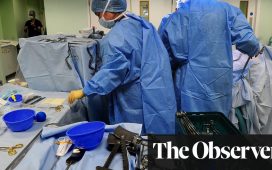
The death of the man who received a groundbreaking pig heart transplant this year occurred from heart failure, concluded doctors at the University of Maryland, where the experimental surgery was performed.
David Bennett, 57, had undergone the experimental surgery in January. During the procedure, doctors had replaced his heart with one from a gene-edited pig.
Bennett had died on 8 March at the University of Maryland Medical Centre after his condition began deteriorating several days earlier.
While doctors have now concluded that Bennett died of heart failure, they are still investigating the reasons for the failure, reported The Baltimore Sun.
“We are still trying to figure out what went wrong; we don’t have a single answer,” said Dr Muhammad M Mohiuddin, co-leader of the pig heart study and professor of surgery and director of the cardiac xenotransplantation programme at the medical school.
“But we don’t consider this a setback,” he pointed out.
“We consider that he lived through the surgery the first win. When he seemed to be recovering and doing well for two months, we really thought that was a huge success. If we could have identified the reason his heart gave out suddenly, he might have walked out of the hospital.”
Doctors said what made the surgery a success was that, in the weeks following the procedure, Bennett was able to get out of bed, begin rehabilitation and spend time with his family.
His body did not show signs of rejecting the heart, an autopsy noted.
Instead doctors had found a thickening and subsequent stiffening of the heart muscle, perhaps a reaction to a drug used to prevent rejection and infection which could not relax and fill the heart with blood as it was supposed to.
The pig, the heart of which was transplanted into Mr Bennet, was reportedly raised by biotech company Revivicor, which altered the pig’s genome to reduce the risk of rejection.
Bennett may have died by a pig virus called porcine cytomegalovirus, a preventable infection linked to devastating effects on transplants, suggested an MIT Technology Review report from May this year.
In a statement, the US Food and Drug Administration (FDA) said such transplants will be considered on a “case by case basis” in the future.
“Overall, FDA will not allow an investigational product to be used unless it believes that such risks are appropriately minimised and acceptable for the clinical situation,” the FDA spokesperson was quoted as saying by The Baltimore Sun.
“Because of the potentially serious public health risks of possible zoonotic infections, FDA has instituted policies such as long-term patient monitoring and prohibitions against blood donation to mitigate against the risk of infectious disease transmission.”
About 110,000 Americans are waiting for an organ transplant, with more than 6,000 dying annually while they’re on the list, according to federal government data.